Case
A 51-year-old male with known hepatocellular carcinoma (HCC) recently underwent successful transarterial chemoembolization of a segment VII liver lesion. The patient was admitted to the hospitalist service for overnight observation. Soon after being sent to the floor, he developed a large mass in his right groin, with associated erythema and tenderness. Upon examination, the radiology resident on call found a 3-cm round red hematoma near the arterial puncture site.
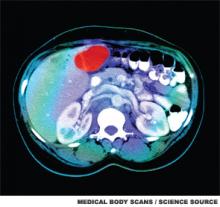
This is a cross sectional image from a CT scan of the abdomen which shows an ill-defined tumor in the liver. The tumor is the red area in the top part of the liver, located next to the gallbladder. The spine (vertebrae) is the white structure near the center of the lower part of the image. To either side of the spine are the kidneys.
Manual pressure was reapplied for 15 minutes, and the mass was circled with a marker. The patient was monitored for an additional day in the hospital with serial blood counts that were stable. Prior to discharge, the hematoma was 1 cm and disappeared by his follow-up, five days later.
Current State of Liver Malignancies
Liver malignancies have increased in incidence over the last decade, from 7.1 to 8.4 per 100,000 people.1 HCC is the most common form of primary liver cancer, with more than one million new cases worldwide each year. While generally more prevalent in countries where hepatitis B is endemic (i.e., China and sub-Saharan Africa), prevalence is increasing in the United States and Europe due to chronic hepatitis C, nonalcoholic steatohepatitis (NASH), and alcoholic cirrhosis. HCC traditionally has had few treatment options, with surgical resection or liver transplantation providing the only potential cures; however, only a minority of patients (10%-15%) are surgical candidates.2,3
Similarly, liver metastasis due to cancers from the gastrointestinal tract and breast are on the rise in developing and developed countries. The National Cancer Institute (NCI) estimates that approximately 50% of patients with colon cancer will have liver metastases at some point in the course of their disease, and only a small number of patients will be candidates for surgical resection.4
In light of the limited treatment options for liver malignancies, alternative treatments continue to be an area of intense research, namely transarterial therapies, the most common of which are briefly described in Table 1.
Puncture Site Complications
Hematoma. Puncture site hematoma is the most common complication of arterial access, with an estimated incidence of 5%-23%.5 The main clinical findings are erythema and swelling at the puncture site, with a palpable hardening of the skin. Pain and decreased range of motion in the affected extremity are common. Severe cases can result in hypotension and tachycardia with an acute drop in hemoglobin. Initial management will involve marking the site to evaluate for change in size as well as applying pressure. Patients should remain in bed, and serial blood counts should be monitored. Simple hematomas may resolve with time; however, more severe cases may require surgical intervention.6,7
Pseudoaneurysm formation. The incidence of pseudoaneurysm after arterial puncture is 0.5%-9%. These primarily arise from difficulty with cannulation of the artery and inadequate compression after vascular sheath removal. Signs of pseudoaneurysm are similar to those associated with hematoma; however, these will present with a palpable thrill or possibly a bruit on auscultation. Ultrasound is used for diagnosis. As with hematoma, bed rest and close monitoring are important. More severe cases may require surgical intervention or thrombin injection.5,8
Infection: Puncture site infection is rare, with incidence around 1%. Pain, swelling, and erythema, in combination with fever and leukocytosis, should raise suspicion for infection. Treatment typically involves antibiotics.