Case
A 66-year-old man with diabetes mellitus type 2 and hypertension underwent left total knee replacement. Several hours after surgery, the patient developed atrial fibrillation (AF). He was asymptomatic, and reversible causes of AF were ruled out. Approximately 18 hours later, he spontaneously reverted back to sinus rhythm. Should this patient, who has no known prior history of AF and a CHA2DS2-VASc score of 3, be started on anticoagulation?
Background
Hospitalists are commonly consulted for evaluation and management of postoperative atrial fibrillation (POAF). The incidence of new-onset AF associated with non-cardiac surgery is approximately 2% and may be more frequent in an elderly population.1 The increased adrenergic tone associated with surgery is thought to elicit AF in some patients. POAF has also been associated with positive fluid balance, electrolyte abnormalities, and hypoxemia.2 Some of these patients will spontaneously revert back to sinus rhythm after these issues are reversed. Others will go on to develop chronic or paroxysmal AF that persists indefinitely. It is also likely that some patients with POAF, in fact, already had asymptomatic AF that was simply undetected prior to hospitalization.
Hospitalists are faced with the difficult task of determining which patients with POAF will benefit from either short-term or long-term anticoagulation. This has not been well studied in postsurgical patients, in contrast to medical patients in whom stroke risk from AF has been very well-characterized. The decision may be further complicated by bleeding risk (associated with either some surgeries or with patient-dependent factors).3
It is worth noting that following major cardiac or thoracic surgery, POAF is common; the incidence ranges from 10% to 60%. In these cases, POAF may be triggered by transient atrial ischemia or by postoperative inflammation and may have a different natural history from POAF in non-cardiac surgery patients in terms of both reversibility and stroke risk. More retrospective data are available regarding cardiothoracic surgery patients.
Previous American Heart Association (AHA) and American College of Cardiology (ACC) guidelines stated that POAF lasting longer than 48 hours warranted anticoagulation. This recommendation was removed from the newest update. The 2014 updated AHA/ACC guidelines are less absolute and now state only that “it is reasonable to administer antithrombotic medication in patients who developed postoperative AF, as recommended for nonsurgical patients” (Level of Evidence: B) in regard to cardiothoracic surgery.4
There is no specific recommendation regarding POAF for non-cardiac surgery patients. The current guidelines are likely purposefully vague due to the lack of direct evidence. The following is a review of the existing literature and a suggested approach to anticoagulation in POAF.
Review
How common is postoperative atrial fibrillation? New-onset AF during hospitalization is known to occur in association with many acute conditions including surgery, infection, and myocardial infarction. About half of the cases of in-hospital new-onset AF are associated with surgery. AF is more commonly seen in surgery that involves the thoracic cavity and cardiac structures. In a cross-sectional epidemiologic study of 22 million patients in California, 20.8% of patients undergoing cardiac surgery developed POAF compared with only 1.3% of patients undergoing non-cardiac surgery.5 A smaller study of non-cardiac surgery patients found a 30-day POAF incidence of 0.37%.2
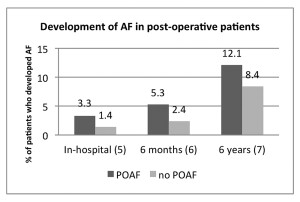
Does postoperative atrial fibrillation increase the short-term risk of stroke? A major concern in AF is the risk of stroke. It is well-established that prolonged or recurrent AF increases the risk for stroke over months or years, but do short episodes of POAF increase stroke risk to a significant degree? Most of the studies in the literature focus on perioperative stroke risk specifically in cardiothoracic surgery. A prospective study of 4,000 patients undergoing cardiac surgery found that the in-hospital postoperative stroke risk was 3.3% in patients with POAF compared to 1.4% in patients without POAF (P<0.01).6 Similar outcomes were seen in a VA study looking at patients who underwent open heart surgery: Stroke risk was 5.3% at six months in POAF patients compared to 2.4% in those without POAF.7 Another study of coronary artery bypass graft (CABG) patients with a follow-up of almost six years showed a stroke risk of 12.1% in POAF patients compared to 8.4% in those without POAF.8
