Unassigned, Undocumented Inpatients Present Challenges; Some Hospitalists Have Solutions
Hospitalists are charged with giving the best of care and treatment, regardless of whether or not a patient is insured or has a PCP to transition to after discharge. But patients who do not have insurance or a PCP pose many challenges to hospitalists, as well as the healthcare systems they work in. Although some hospitals and health systems have found ways to address these challenges, issues persist, with high costs to care for these patients topping the list. In 2013, the cost of community hospitals’ uncompensated care climbed to $46.4 billion.1
Typically, undocumented and unassigned patients face many social and economic challenges. Many of these patients are unemployed or work as independent contractors without employer-offered health insurance. Some have multiple jobs, can’t take time off from work for doctor appointments, or are undocumented workers.
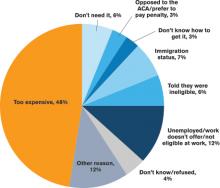
More patients have acquired health insurance in recent years as a result of the Affordable Care Act (ACA) and Medicaid expansion; however, some eligible people never complete the necessary forms.
With or without insurance, some patients don’t establish primary care because they have been healthy, have difficulty navigating the healthcare system, lack transportation, or desire more culturally tailored care. Some Medicare and Medicaid patients don’t have a PCP in their community who accepts these programs.
Treatment Challenges
Uninsured patients often are sicker and have more complex conditions than those with insurance, according to Beth Feldpush, DrPH, senior vice president of policy and advocacy at the nonprofit trade group America’s Essential Hospitals, which is based in Washington, D.C., and represents 250 safety net hospitals throughout the U.S.
“Because they can’t afford regular preventive and primary care, they forgo needed healthcare services until their conditions worsen and they require costly hospital care,” says Dr. Feldpush. Uninsured patients often lack the resources for follow-up care to help them recover and stay well. She says more than half of all inpatient discharges and outpatient visits at her groups’ hospitals are for uninsured or Medicaid patients.
When an uninsured patient is discharged from the hospital, finding follow-up care can be difficult.
“Their ability to get an appointment to see a PCP is extremely limited, because many providers don’t see patients without health insurance,” says Scott Sears, MD, MBA, chief clinical officer of Tacoma, Wash.-based Sound Physicians. Dr. Sears notes that in some hospitalist programs, as many as 40% of hospitalized patients lack insurance. “But without secured follow-up care, hospitalists are hesitant to send patients home, because they could relapse.”
Typically, these patients are not completely well and should be transferred to a skilled nursing or hospice facility; however, many facilities won’t accept them without insurance. Often, these patients need a PCP to monitor them with laboratory tests and other follow-up tests, to prescribe and monitor medications, and to ensure that they are following their plan of care.
At some medical facilities, subspecialists who consult on patients may screen them and refuse to see anyone without health insurance.
“So even though some patients may need subspecialty support, they may not have access to it,” Dr. Sears says. “While some patients without insurance qualify for Medicaid or other programs, due to the amount of paperwork and time to enroll, they end up staying in the hospital even though they are ready for discharge.”
Transitional Challenges
Most patients admitted to the hospital either have exacerbations of chronic conditions or a new diagnosis. “It’s rare to hospitalize a patient with a discrete illness that wouldn’t need care after discharge, so having a robust PCP partner is critical to a patient’s health,” says Honora Englander, MD, medical director of the Care Transitions Innovation (C-TRAIN) program at Oregon Health and Science University (OHSU) in Portland. For many patients, psychosocial complexity complicates their transition out of the hospital. An effective system needs to address a patient’s mental health, housing, and other social needs.