Republican or Democrat, Americans vote for face masks
Most Americans support the required use of face masks in public, along with universal COVID-19 testing, to provide a safe work environment during the pandemic, according to a new report from the Commonwealth Fund.
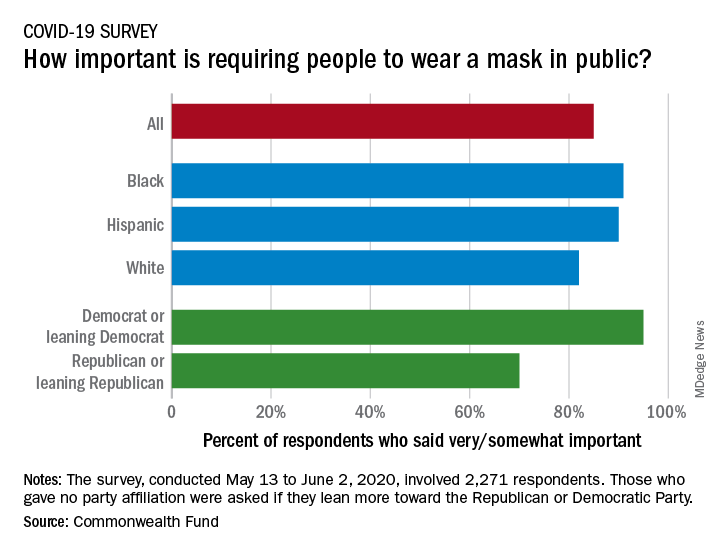
Results of a recent survey show that 85% of adults believe that it is very or somewhat important to require everyone to wear a face mask “at work, when shopping, and on public transportation,” said Sara R. Collins, PhD, vice president for health care coverage and access at the fund, and associates.
In that survey, conducted from May 13 to June 2, 2020, and involving 2,271 respondents, regular COVID-19 testing for everyone was supported by 81% of the sample as way to ensure a safe work environment until a vaccine is available, the researchers said in the report.
Support on both issues was consistently high across both racial/ethnic and political lines. Mandatory mask use gained 91% support among black respondents, 90% in Hispanics, and 82% in whites. There was greater distance between the political parties, but 70% of Republicans and Republican-leaning independents support mask use, compared with 95% of Democrats and Democratic-leaning independents, they said.
Regarding regular testing, 66% of Republicans and those leaning Republican said that it was very/somewhat important to ensure a safe work environment, as did 91% on the Democratic side. Hispanics offered the most support by race/ethnicity, with 90% saying that testing was very/somewhat important, compared with 86% of black respondents and 78% of white respondents, Dr. Collins and associates said.
Two-thirds of Republicans said that it was very/somewhat important for the government to trace the contacts of any person who tested positive for COVID-19, a sentiment shared by 91% of Democrats. That type of tracing was supported by 88% of blacks, 85% of Hispanics, and 79% of whites, based on the polling results.
The survey, conducted for the Commonwealth Fund by the survey and market research firm SSRS, had a margin of error of ± 2.4 percentage points.