![1]()
News
CDC issues COVID-19 vaccine guidance for underlying conditions
December 29, 2020
The CDC guidance had specific information for people with HIV, weakened immune systems, and autoimmune conditions such as Guillain-Barré syndrome and Bell’s palsy.
News
CDC identifies next priority groups for COVID-19 vaccine
December 21, 2020
ACIP said the next priority group, Phase 1b, should consist of what it called frontline essential workers, a group of about 30 million, and adults aged 75 years and older, a group of about 21 million.
News
Second COVID-19 vaccine ready for use, CDC panel says
December 20, 2020
Moderna’s COVID-19 vaccine — the second now cleared for emergency use in the United States — was endorsed by the Advisory Committee on Immunization Practices (ACIP) of the Centers for Disease Control and Prevention (CDC) on Dece...
![1]()
News
FDA grants emergency use for Moderna COVID-19 vaccine
December 19, 2020
“It’s incumbent upon all us healthcare professionals to put ourselves out there as supporting this vaccine and supporting people getting it.”
![1]()
News
Moderna COVID-19 vaccine wins decisive recommendation from FDA panel
December 18, 2020
The advisers and FDA staff also honed in on several key issues with COVID-19 vaccines, including the challenge of having people in the placebo groups of studies seek to get cleared vaccines.
![1]()
News
COVID-19 vaccines: Safe for immunocompromised patients?
December 17, 2020
Data are limited thus far, and the safety and efficacy of the COVID-19 vaccines in immunocompromised patients remain unknown.
![1]()
News
Coronavirus has infected over 2% of U.S. children
December 15, 2020
AAP president: “In many areas, our health care system is terribly overburdened. The vaccine will not solve this overnight.”
![1]()
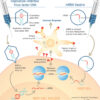
Opinion
Understanding messenger RNA and other SARS-CoV-2 vaccines
December 14, 2020
Technological advances make these new and revolutionary vaccines a reality.
News
CDC panel recommends Pfizer’s COVID-19 vaccine for people 16 and over
December 13, 2020
ACIP panelists said clinicians need more guidance on whether to use the vaccine in pregnant or breastfeeding women.
![1]()
News
FDA OKs emergency use of Pfizer COVID-19 vaccine
December 12, 2020
“Vaccinating healthcare workers, in particular, will be a model for the general public.”